Medical wire
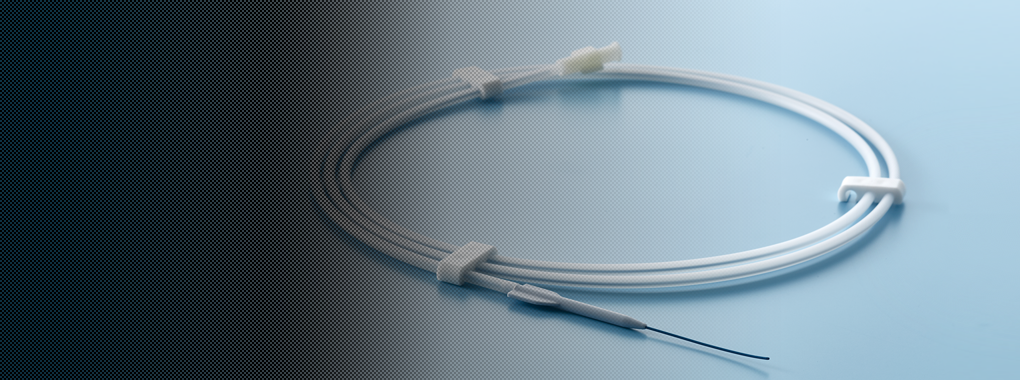
This wire is a medical application of our special steel wire technologies, which have a proven track record of reliability in the automotive and other industries. In this new area of business, we intend to contribute to the health of people around the world.

History of medical business
| April 2024: | Integrate the trading functions into Nippon Steel SG Wire Co., Ltd. |
|---|---|
| April 2019: | Restructured SKK Technology Co., Ltd. business organization: •Incorporated medical business, including sales, technology and quality assurance,into Nippon Steel SG Wire Co., Ltd. •Transferred medical manufacturing to NSSG Partners Co., Ltd. •Transferred trading company functions to SKK Technology Co., Ltd. |
| September 2018: | Upgraded certification to ISO13485: 2016 |
| September 2018: | Updated from manufacturing license to registration |
| March 2017: | Introduced clean room manufacturing |
| June 2016: | Acquired ISO13485: 2003 certification |
| July 2014: | Established Medical Wire Business Division to strengthen our medical business |
| July 2009: | Merged three related companies co-located at the site of Narashino Plant, Suzuki Metal Industry Co., Ltd. to establish SKK Technology Co., Ltd. |
| 1998: | Acquired medical device manufacturing license |
| 1983: | Began manufacturing of coil type guide wires, mainly by affiliated companies |
| 1961: | Suzuki Metal Industry Co., Ltd. acquired shares of former Nitto Kinzoku Kogyo Co., Ltd. |
ISO13485: 2016 (quality management system standard for medical devices)
We have acquired ISO13485: 2016 certification, the quality management system standard for medical devices. Our Medical Wire Business Division designs, develops and manufactures guide wire components that are used every day in catheter treatments. We will continue to provide such safe, high quality products and services to our customers.
| Applicable standard | ISO13485:2016 |
| Scope of certification | Design and manufacturing of unsterilized guide wires |
| Examination and registration organization | SGS Japan Inc. |
| Certification number | JP16/819942342 |
| Certification date | June 23, 2016 |
| Acquisition site | Medical Wire Business Division, Nippon Steel SG Wire Co., Ltd. |


Medical device manufacturing industry registration
We are a medical device manufacturer registered according to the provisions of Article 23-2-3, Paragraph 1 of the Pharmaceuticals and Medical Devices Act. We are fully capable of manufacturing and supplying medical equipment and parts for medical devices. Please feel free to contact us.
| Registration number | 12BZ200221 |
| Company name | Nippon Steel SG Wire Co., Ltd. |
| Manufacturer name | Nippon Steel SG Wire Co., Ltd. |
| Manufacturer location | 7-5-1 Higashinarashino, Narashino-shi, Chiba-ken, 275-8577, Japan |
| Period of validity | April 1, 2024 — March 31, 2029 |
| Registration rights holder | Toshihito Kumagai |

Dealing products
Taper processing
 |
Coil spring
 |
Straight processing line, Special processing
 |
